Making the invisible visible through research
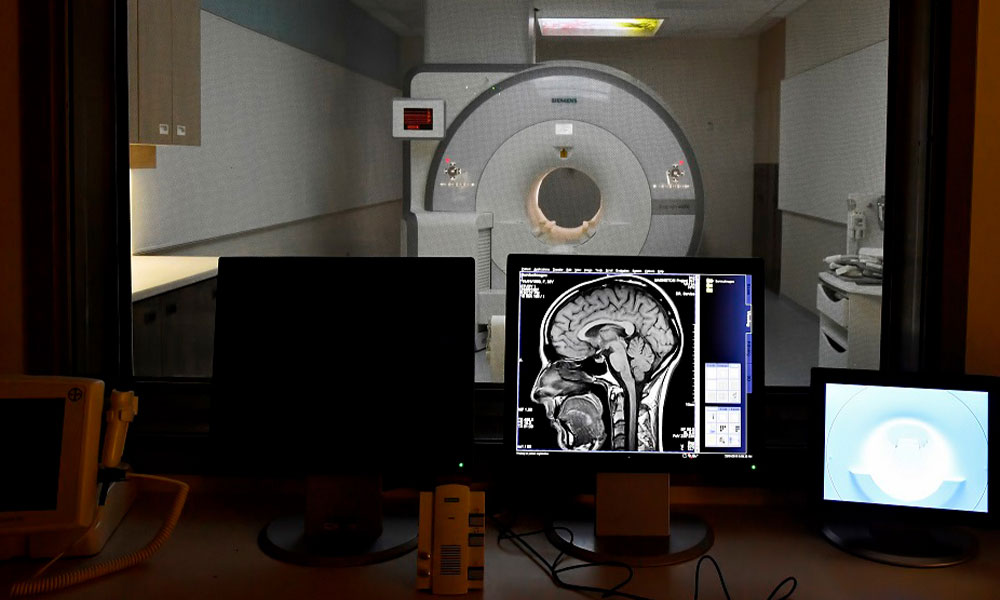
The Royal’s Brain Imaging Centre (BIC) is a leader in neuroimaging, dedicated to advancing mental illness research through specialized technology and expertise. Featuring a cutting-edge Positron Emission Tomography - Magnetic Resonance Imaging (PET-MRI) scanner dedicated to research, we combine multiple type of imaging to give researchers a complete picture of the brain’s structure, function, chemistry and electrical activity. This unique view helps researchers understand how the brain is disrupted by mental illness and addiction.
A decade of growth: expanding our impact in research
Since 2016, we have completed more than 6,000 research scans. We are now conducting well over a thousand scans annually, reflecting the expanding needs of the research community we serve. As of 2025, we have supported more than 72 research protocols and collaborated with more than 40 researchers across academia, healthcare, industry and government including the University of Ottawa, Carleton University, The Ottawa Hospital, CHEO Research Institute and Bruyère Health Research Institute. Our strong partnership with the University of Ottawa Heart Institute Radiochemistry Lab is key to the success of the PET research program at The Royal.
Leveraging the BIC to support your research
The BIC is available to research teams across all universities, hospitals and research institutes in the Ottawa region. In addition to our mandate to accelerate mental illness discoveries through neuroimaging, the facility is available for all whole-body imaging needs. For example, we have experience in cardiac, MSK and industry-sponsored clinical trials. The BIC offers flexible scanner availability and provides wrap-around services to our users including protocol and sequence development, data management and image analysis. For more information on launching your research at the BIC, please contact the facility manager, Tram Nguyen, (nguyentram@theroyal.ca).
Participate in Neuroimaging Research
Many research studies happening at The Royal involve brain imaging. Participation in research contributes directly to new discoveries at The Royal and beyond.